What do investors think about antibiotics companies? Not much. Paratek, at a price of just $5.42 has a 36% short ratio. That is, 36% of tradable shares are being shorted by skeptical investors. Market caps under $200 million do not bold well either. Melinta is selling four antibiotics – they made $96 million in total revenue in 2018. Anyone who doubts that the antibiotic market is broken needs only ask the investors.
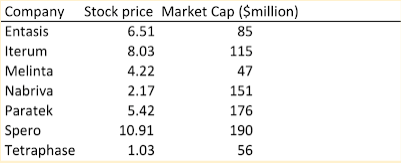
As I noted in my previous blog, it is unlikely that we will see a significant pull incentive this year. Investors, I’m told, prefer a direct reimbursement above the DRG for expensive new antibiotics as described in the DISARM act of 2018. But hospitals may well balk at being reimbursed for an expensive product they may not use. I’m not sure the investors have asked the hospitals what they think. In the UK, meanwhile, they are still considering the added value of antibiotics to their healthcare system. But, it seems, they have already decided on a value or on the price of a market entry reward, or whatever pull incentive they may or may not apply.
I first heard this number from folks at Wellcome last fall – £50 million. By strange coincidence, in the recent opinion piece by Jeremy Farrar of the Wellcome Trust, he notes that the fair share for the UK of a $1billion reward would be 5% or $50million (that’s even less than £50million).First – I believe a more workable number for a market entry reward is $2 billion, especially if we want to attract large pharma and investors back into the space. Secondly, I’m not sure that a $50 million contribution by the UK would be considered a good thing by investors – that small a number might even be a disincentive. The only way that approach will work is if 20-40 other countries join in – a scenario that seems hardly likely. This approach seems less like leadership and more like something else.
On a possibly brighter note, the WHO recently released a report on the antibiotic resistance crisis. One key conclusion was:
Additional effort, investments and incentives are needed to spur innovation in antimicrobial medicines, diagnostics, vaccines, waste management tools, safe and effective alternatives to antimicrobials and alternative practices, as well as operational and implementation research, in human, animal and plant health.
In typical WHO fashion, this leaves much to be desired in terms of providing for a significant global pull incentive for antibiotic R&D.
All of this gets me back to where I was in my last blog. What can we do while we wait for someone to lead with a significant pull incentive? We lobby the Infectious Diseases Society of America (IDSA) do its job! I’m sorry – but the current situation is simply unacceptable to me as an infectious diseases physician. I just reviewed the IDSA/American Thoracic Society guidelines on treatment of ventilator-associated and hospital-acquired pneumonia.
These are remarkably well thought out with a great deal of literature research for the year of their publication -2016. But much has changed since then. Would the IDSA/ATS still stand by their statement that “We did not identify any RCTs assessing colistin as empiric therapy for VAP, but a systematic review and meta-regression of observational studies comparing colistin to other antibiotics found no differences in clinical response rates, mortality, or nephrotoxicity [184]?” (Personally, I have instructed my family to threaten any physician contemplating treating me with colistin/polymyxin with a malpractice suit!)
I worked on guidance from IDSA and the Society for Health Care Epidemiology in the 1990s. The guidance appeared three years after we started our work and was not updated for 10 years. Of course, I like to think our guidance document was so far-sighted that it did not need updating – but that’s just me. The bureaucratic wheels grind slowly, especially when there are two different organizations trying to get things approved and published. I think the Societies need to find a way to respond to rapidly changing data to update physicians in a real-time way with updated guidance. This would go a long way to alter physician behavior and get our patients the right antibiotics when they need them most. It would also spur hospitals to have the right antibiotics on their formularies and, in consequence, would provide one step towards improving the antibiotic marketplace.
No comments:
Post a Comment