By Alex Berezow — May 12, 2018
Bacteria come in two very broad categories based on the structure of their cell walls, the outer region that gives the cells shape and integrity. The cell walls of Gram-positive bacteria consist of a membrane surrounded by a thick layer of sugar and protein, while the cell walls of Gram-negative bacteria consist of a membrane surrounded by a second membrane.
This fundamental anatomical difference has a profound medical implication: The types of antibiotics that can kill Gram-positive bacteria are likely ineffective against Gram-negative bacteria and vice versa. A major reason is the cell wall: An antibiotic may be able to penetrate one type but not the other. This is why, for instance, that the drug vancomycin is prescribed to treat Gram-positive infections, such as Staphylococcus, but is completely useless against Gram-negative infections, such as E. coli.
Finding new ways to sneak antibiotics past these cell wall barriers is one way that microbiologists are fighting antibiotic resistance. Now, researchers have shown that a synthetic "double Trojan horse" drug can fool bacteria into willingly accepting a toxic antibiotic, essentially causing them to commit suicide.
Fool Me Once
Like all cells, bacteria require nutrients to survive. Iron ions are not easy to come by, so some bacteria have evolved an elaborate mechanism to gather them. They secrete specialized iron-harvesting molecules into the environment and, upon binding an iron ion, the molecules are recaptured and actively transported back into the bacteria via specific protein channels. By hijacking this process, an iron-harvesting molecule can be turned into a deadly Trojan horse.
This is done by sticking an antibiotic onto an iron-harvesting molecule, creating what is known as a sideromycin. When the unsuspecting bacterium gobbles it up, expecting a healthy iron ion, it also gets a dose of antibiotic. These sideromycins have been synthesized by scientists and, surprisingly, by clever bacteria as well. (Microbes create antibiotics as a form of biological warfare against other microbes; they don't create them for our benefit.)
As might be expected, some bacteria have evolved defense mechanisms against such tricks. There are multiple mechanisms by which bacteria can evade antibiotics, and one of them involves destruction of the antibiotic. The Gram-negative bacterium Acinetobacter baumanii -- colloquially known as "Iraqibacter" and which has gained notoriety for causing antibiotic-resistant infections in American troops -- possesses an enzyme that destroys certain types of antibiotics.
So, scientists incorporated a second antibiotic into the Trojan horse molecule. Now, when the enzyme wielding bacterium destroys the first antibiotic, it unwittingly unleashes the second antibiotic, for which it has no defense. (By analogy, it would be as if the act of defusing a letter bomb detonated a second bomb.) In other words, the bacterium was tricked into committing suicide by a "double Trojan horse."
Fool Me Twice
The diagram below (which has been modified from the original) shows the mechanism by which the double Trojan horse drug works.
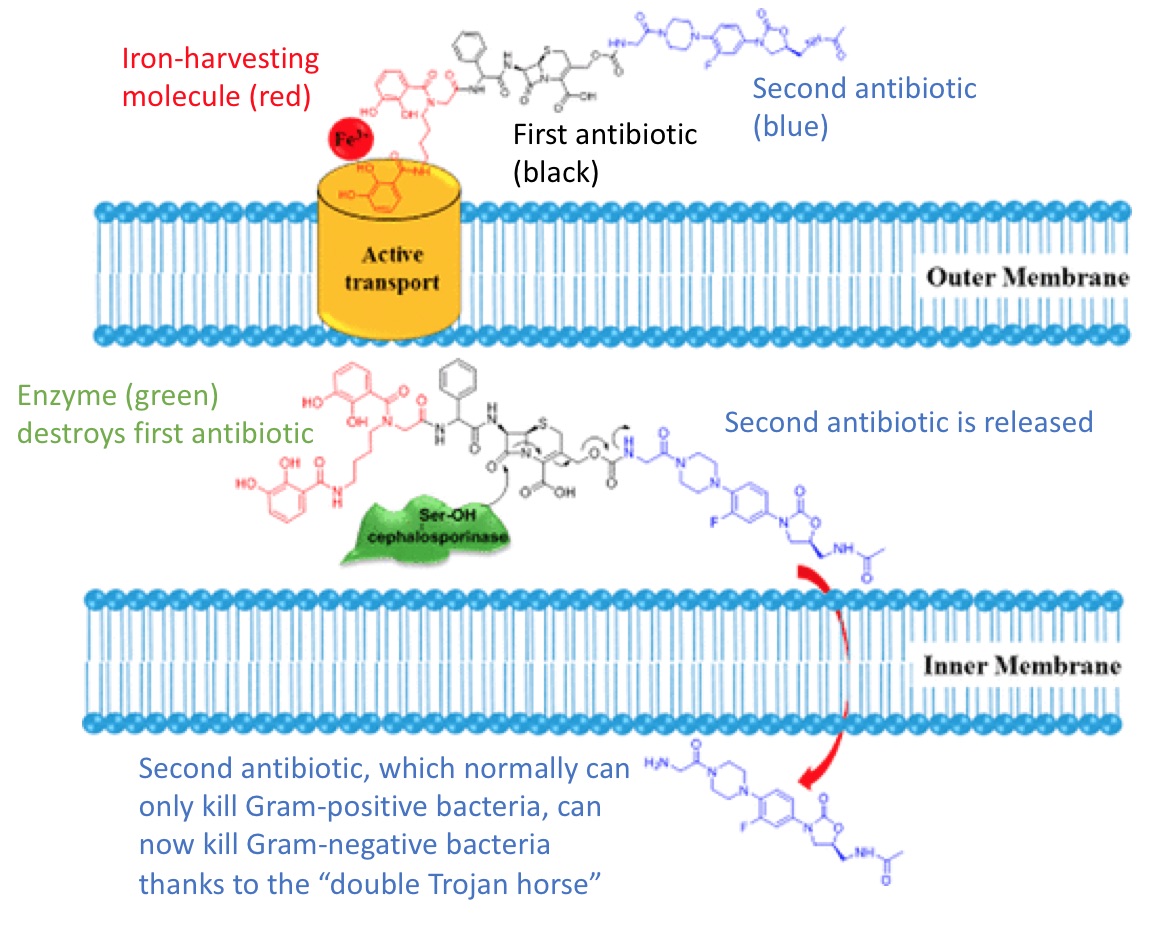 The bacterium, in this case the Gram-negative Acinetobacter, actively transports the double Trojan horse molecule across its first membrane. The bacterium is anticipating an iron ion, but it also gets two antibiotics along with it. It has an enzyme capable of destroying the first antibiotic (in this case, cephalosporin). But the act of destroying the first antibiotic releases the second antibiotic (in this case, oxazolidinone).
The bacterium, in this case the Gram-negative Acinetobacter, actively transports the double Trojan horse molecule across its first membrane. The bacterium is anticipating an iron ion, but it also gets two antibiotics along with it. It has an enzyme capable of destroying the first antibiotic (in this case, cephalosporin). But the act of destroying the first antibiotic releases the second antibiotic (in this case, oxazolidinone).
Normally, oxazolidinone is incapable of killing Gram-negative bacteria because it cannot get past the two membranes. But through this double Trojan horse mechanism, scientists have devised a way to sneak it in. Ironically, from the bacterium's perspective, the very enzyme that it uses to protect itself from antibiotics becomes complicit in its own demise.
Source: Rui Liu, Patricia A. Miller, Sergei B. Vakulenko, Nichole K. Stewart, William C. Boggess, and Marvin J. Miller. "A Synthetic Dual Drug Sideromycin Induces Gram-Negative Bacteria To Commit Suicide with a Gram-Positive Antibiotic." J. Med. Chem. 61 (9): 3845-3854. Published: 19-March-2018 DOI: 10.1021/acs.jmedchem.8b00218
Finding new ways to sneak antibiotics past these cell wall barriers is one way that microbiologists are fighting antibiotic resistance. Now, researchers have shown that a synthetic "double Trojan horse" drug can fool bacteria into willingly accepting a toxic antibiotic, essentially causing them to commit suicide.
Fool Me Once
Like all cells, bacteria require nutrients to survive. Iron ions are not easy to come by, so some bacteria have evolved an elaborate mechanism to gather them. They secrete specialized iron-harvesting molecules into the environment and, upon binding an iron ion, the molecules are recaptured and actively transported back into the bacteria via specific protein channels. By hijacking this process, an iron-harvesting molecule can be turned into a deadly Trojan horse.
This is done by sticking an antibiotic onto an iron-harvesting molecule, creating what is known as a sideromycin. When the unsuspecting bacterium gobbles it up, expecting a healthy iron ion, it also gets a dose of antibiotic. These sideromycins have been synthesized by scientists and, surprisingly, by clever bacteria as well. (Microbes create antibiotics as a form of biological warfare against other microbes; they don't create them for our benefit.)
As might be expected, some bacteria have evolved defense mechanisms against such tricks. There are multiple mechanisms by which bacteria can evade antibiotics, and one of them involves destruction of the antibiotic. The Gram-negative bacterium Acinetobacter baumanii -- colloquially known as "Iraqibacter" and which has gained notoriety for causing antibiotic-resistant infections in American troops -- possesses an enzyme that destroys certain types of antibiotics.
So, scientists incorporated a second antibiotic into the Trojan horse molecule. Now, when the enzyme wielding bacterium destroys the first antibiotic, it unwittingly unleashes the second antibiotic, for which it has no defense. (By analogy, it would be as if the act of defusing a letter bomb detonated a second bomb.) In other words, the bacterium was tricked into committing suicide by a "double Trojan horse."
Fool Me Twice
The diagram below (which has been modified from the original) shows the mechanism by which the double Trojan horse drug works.

Normally, oxazolidinone is incapable of killing Gram-negative bacteria because it cannot get past the two membranes. But through this double Trojan horse mechanism, scientists have devised a way to sneak it in. Ironically, from the bacterium's perspective, the very enzyme that it uses to protect itself from antibiotics becomes complicit in its own demise.
Source: Rui Liu, Patricia A. Miller, Sergei B. Vakulenko, Nichole K. Stewart, William C. Boggess, and Marvin J. Miller. "A Synthetic Dual Drug Sideromycin Induces Gram-Negative Bacteria To Commit Suicide with a Gram-Positive Antibiotic." J. Med. Chem. 61 (9): 3845-3854. Published: 19-March-2018 DOI: 10.1021/acs.jmedchem.8b00218

No comments:
Post a Comment